May 10, 2017
CSOFT provides Didi Chuxing localization services
Didi Chuxing, China’s biggest ride-share company, has released its app services in English, marking March 8th as a life-changing day for foreigners living in China.
After Didi acquired Uber in 2016, international residents and travelers in China lost their primary option to use ride-share services, as Didi’s app was only available in Chinese and prohibited the use of international credit cards. However, with an initial update available in Shanghai, Beijing, and Guangzhou, users now have the option of an English interface, real-time translation to communicate with drivers, and even the ability to connect international credit cards.
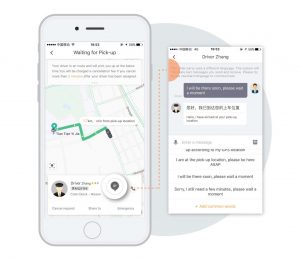
between English and Chinese.
The release of the popular app has been long-awaited by expatriates and travelers in China, and Didi’s move in providing English services is pivotal in their journey to global expansion. As part of its global strategy, Didi announced that the “internationalization of mobility services in China is a crucial link in Didi’s broader global strategy [and] as an international economic and cultural hub, China increasingly attracts inbound foreign tourists, business travelers and expatriates.”
With overseas partnerships already in Southeast Asia and India, Didi announced that it had raised $5.5 billion in funds for its worldwide ambitions, bringing its private valuation to over $50 billion dollars. This has made it China’s most valuable startup in history and second in the world only to Uber.
Marking only the beginning of its international expansion, Didi’s decision to partner with CSOFT International, a leader in localization and globalization services, is proving to be the correct strategy for its global aspirations.
About CSOFT Health Sciences
CSOFT Health Sciences, leaders in clinical trial localization, provides AI/ML-enabled medical translation services for all phases of the drug and medical device product lifecycle, from development to post-launch. We also specialize in DCT solutions, linguistic validation, and CTD/eCTD submissions with the FDA, EMA, and NMPA. Our operations are certified in ISO 17100:2015, ISO 9001:2015, and ISO 13485:2016, ensuring our customized solutions meet the rigorous regulatory requirements of MMA, NDA, CTA, and Medical Device Application submissions. www.csoftintl.com

